Bictegravir 1611493-60-7
Descriptio
Bictegravir nova est, potens inhibitor HIV-1 integrae cum IC50 7.5 nm.
in Vitro
Bictegravir (BIC) vetat trans- lationem actionem cum IC50 inter 7.5± 0.3 nM. Relativum ad suum inhibitionis trans- litus activitatem, Bictegravir multo infirmior est inhibitor of 3 ..-processing actio HIV-I IN, cum IC50 de 241±51 nM. Bictegravir auget cumulum 2-LTR circulorum ~5-plicarum relativum ad ludibrium tractatum imperium ac quantitatem productorum integrationis authenticae in cellulis infectis per 100-duplicem reducit. Bictegravir potenter vetat replicationem HIV-1 in utroque MT-2 et MT-4 cellularum cum EC50s 1.5 et 2.4 nM, respective. Bictegravir effectus antivirales potentes exhibet lymphocytes tum primarios CD4+ T et macrophages monocyto-deductum, cum EC50s 1.5.±0.3 nM et 6.6 "±4.1 nM, respective, quae cum valoribus in lineis T-cellulis habitis comparantur[1].
MCE independenter subtilitatem horum methodorum non confirmavit. Tantum referendi sunt.
| NCT Number | Sponsor | Conditio | Satus Date | Phase |
| NCT03998176 | Universitas Nebraska|Gilead Sciences | HIV-1-infectio | October 9, 2019 | Phase 4 |
| NCT03789968 | Thomas Jefferson University|University of Maryland, College Park| University Health in Indiana|The Brooklyn Hospital Center|University Illinois at Chicago|Nova Southeastern University|University of California, San Francisco | HIV+AIDS | Septembris 1, 2019 | |
| NCT04249037 | University of Colorado, Denver|Gilead Sciences | HIV+AIDS | Martii 1, 2020 | Non Lorem |
| NCT04132674 | Vancuverium Infectious Diseases Centre | Humana immunitas Virus I Infectio|Drug Use | November 26, 2018 | Phase 4 |
| NCT04054089 | Cristina Mussini Universitas Mutinensis et Regii Lepidi | HIV Infectiones | September 2019 | Phase 4 |
| NCT04155554 | Azienda Ospedaliera Universitaria Senese | Catholic University of the Holy Heart | Ospedale Policlinico San Martino | | HIV-1-infectio | die 29 mensis Ianuarii anno 2020 | Phase 3 |
| NCT02275065 | Gilead Sciences | HIV-1 Infectio | October 2014 | Phase 1 |
| NCT03711253 | Universitas Southern California | Acutus HIV Infectio | October 14, 2019 | Phase 4 |
| NCT02400307 | Gilead Sciences | HIV | April 17, 2015 | Phase 1 |
| NCT03499483 | Fenway Community Health | HIV Prevention | Die 24 mensis Ianuarii, 2019 | Phase 4 |
| NCT03502005 | Mediterranei Research Group, Inc.|Gilead Sciences | Virus immunis | Martii 1, 2018 | Phase 4 |
Chemical structure
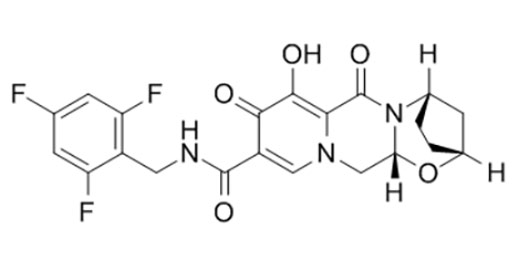





Rogatio18Qualitas Constantia Aestimatio inceptis quae probaverunt4et6incepta approbantes.

Provectus qualitas internationalis administrationis systematis solidum fundamentum in venditionibus posuit.

Qualitas vigilantia percurrit totum vitae cyclum producti, ut qualitatem et therapeuticum effectum curet.

Negotiis professionales regulares turmas sustinet qualitatem postulatorum in applicatione et adnotatione.


Korea Countec Bottled Packaging linea


Taiwan CVC Bottled Packaging Line


Italia CAM Board Packaging Line

German Fette Compacting Machine

Iaponia Viswill Traba Detector

DCS Control Room





