Captopril
Descriptio
Captopril (SQ-14534) est potens, auctor inhibitor angiotensin-conversionis enzyme (ACE).
In vitro
Captopril (SQ-14534) ostensum est, similes morbiditatis et mortalitatis beneficia erga aegros diureticos et beta-blockersos in hypertensivis habere.Captopril (SQ-14534) ostensum est progressionem nephropathiae diabeticae morari, et enalapril et lisinoprilis impedire progressionem nephropathiae in aegris normoalbuminicis cum diabete[1].Ratio aequimolaris cis et trans status Captoprilis (SQ-14534) in solutione existit et enzyme solum eligat statum trans- inhibitoris, qui exhibet complementarium architecturae et stereo- electronicae cum suo substrato ligaturae sulcus[2].
MCE independenter subtilitatem horum methodorum non confirmavit.Tantum referendi sunt.
Orci iudicio
| NCT Number | Sponsor | Conditio | Satus Date | Phase |
| NCT03179163 | Penn State University|National Heart, Lung, and Sanguinis Institutum (NHLBI) | Hypertension, Essentiale | Die 20 Iulii 2016 | Phase 1|Phase 2 |
| NCT03660293 | Tanta University | Diabetes Mellitus, Typus 1 | Aprilis 1, 2017 | Non pertinet |
| NCT03147092 | Centro Neurológico de Pesquisa e Reabiitaci, Brazil | Hypertension|Impressio sanguinis | Februarii 1, 2018 | Mane Phase 1 |
| NCT00252317 | Rigshospitalet, Denmark | Aorta Stenosis | Novembris 2005 | Phase 4 |
| NCT02217852 | West Sinis Hospitalis | Hypertension | August 2014 | Phase 4 |
| NCT01626469 | Brigham ac Women's Hospitalis | Typus 2 Diabetes Mellitus | May 2012 | Phase 1|Phase 2 |
| NCT00391846 | AstraZeneca | Cordis Deficio|Ventricularis Praesent, Left | Octobris 2006 | Phase 4 |
| NCT00240656 | Hebei Medical University | Hypertension, Pulmonarium | Octobris 2005 | Phase 1 |
| NCT00086723 | Northwestern University|National Cancer Institute (NCI) | Adultus Solidus Tumor, Protocollum Imprimis | Iulii 2003 | Phase 1|Phase 2 |
| NCT00663949 | Scirasium University of Sciences Medical | Diabetic Nephropathia | Februarii 2006 | Phase 2|Phase 3 |
| NCT01437371 | University Hospital, Clermont-Ferrand|Servier|LivaNova | Cor defectum | August 2011 | Phase 3 |
| NCT04288700 | Ain Shams University | Infantile Hemangioma | October 1, 2019 | Phase 4 |
| NCT00223717 | Vanderbilt University|Vanderbilt University Medical Center | Hypertension | Ianuarii 2001 | Phase 1 |
| NCT02770378 | University of Ulm | Glioblastoma | November 2016 | Phase 1|Phase 2 |
| NCT01761916 | Instituto Materno Infantil Fernando Figueira Prof | Preeclampsia | Ianuarii 2013 | Phase 4 |
| NCT01545479 | Instituto de Cardiologia do Rio Grande do Sul | Morbus renalis | Ianuarii 2010 | Phase 4 |
| NCT00935805 | Hospital de Clinicas de Porto Alegre|Conselho Nacional de Desenvolvimento Científico e Tecnológico | Diabetes Mellitus | | Iulii 2006 |
|
| NCT00742040 | Hospitale pro infirmis Liberi | Morbus cordis | August 2008 | Phase 2 |
| NCT03613506 | Wuhan University | Radiotherapy Side Effectus | Sumens Captopril | die 25 Octobris 2018 | Phase 2 |
| NCT00004230 | Northwestern University|National Cancer Institute (NCI) | Cancer | Octobris 1999 | Phase 3 |
| NCT00660309 | Novartis | Typus 2 Diabetes Mellitus | Aprilis 2008 | Phase 4 |
| NCT00292162 | NHS Maiora Glasguensis et Clyde | Chronica Cordis Deficio|Atrial Fibrillation | Ianuarii 2007 | Non pertinet |
| NCT01271478 | Coordinación de Investigación en Salud, Mexico | Inflammatio|Finis-scaena Renal Disease | August 2009 | Phase 4 |
| NCT04193137 | Chongqing University | Primarius Aldosteronismus | November 30, 2019 |
|
| NCT00155064 | National Taiwan University Hospitalis | Hyperaldosteronism | Iulii 2002 | Phase 4 |
| NCT01292694 | Vanderbilt University|Vanderbilt University Medical Center | Hypertension|Pura autonomica culpa|Multiplex Ratio Atrophy | Martii 2011 | Phase 1 |
| NCT00917345 | National Taiwan University Hospital|Novartis | Primarius Aldosteronismus | Ianuarii 2008 |
|
| NCT00077064 | Radiation Therapy Oncology Group|National Cancer Institute (NCI)|NRG Oncology | Lung Cancer|Pulmonary Complications|Radiation Fibrosis | Iunii 2003 | Phase 2 |
Repono
| Pulvis | -20°C | III annos |
| 4°C | Annis II | |
| In solvendo | -80°C | VI menses |
| -20°C | I mensis |
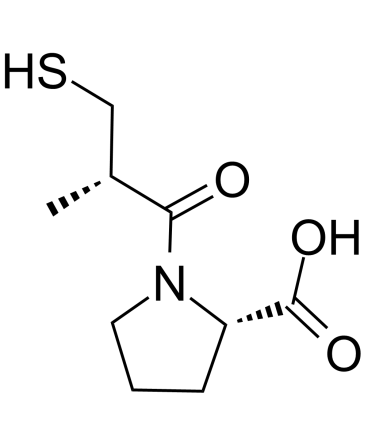
Chemical structure





Rogatio18Qualitas Constantia Aestimatio inceptis quae probaverunt4, et6incepta approbantes.

Provectus qualitas internationalis administrandi ratio solidum fundamentum venditio posuit.

Qualitas vigilantia percurrit totum vitae cyclum producti, ut qualitatem et therapeuticum effectum curet.

Negotiis professionalibus regularibus turmas sustinet qualitatem postulatorum in applicatione et adnotatione.


Korea Countec Bottled Packaging linea


Taiwan CVC Bottled Packaging Line


Italia CAM Board Packaging Line

German Fette Compacting Machine

Iaponia Viswill Traba Detector

DCS Control Room







