Doxycycline Hyclate
Background
Hyclate doxycyclina antibiotica est [1].
Hyclatus doxycyclinus tetracyclinus derivatus est et habet actiones anti-inflammatorii et antimicrobialis. Doxycyclinum replicationem virus dengue in vitro cum modo temperato-dependens inhibet. Valor IC50 est 52.3μM ad 37°C et 26.7μM ad 40°C. Virus dengue inhibet per seriam NS2B-NS3 inhibitionem viri. 60μM doxycyclinum CPE cellularum infectarum DNEV2 reducit [1].
Doxycyclina inhibitor MMP inventus est. Curatio doxycyclina MMP-8 et -9 gradus minuit et expressionem textus MMP-2 et MMP-9. Curatio praeterea cum doxycyclino insigniter reducit incidentiam aneurysmatum intracranialium. Doxycyclinum etiam nuntiavit esse agens anti-inflammatorium secundum suum inhibitionem matricis metalloproteinases. Praeterea doxycyclinus potentem actionem antimalarialem cum IC50 valore 320nM ad 96h in vitro habet [2, 3].
Notae:
[1] Rothan HA, Mohamed Z, Paydar M, Rahman NA, Yusof R. Effectum inhibitorium doxycyclini contra virus replicationis in vitro dengue. Arcus Virol. 2014 Apr;159(4):711-8.
[2] Maradni A, Khoshnevisan A, Mousavi SH, Emamirazavi SH, Noruzijavidan A. Munus matricis metalloproteinases (MMPs) et MMP inhibitores in aneurysmatibus intracranialibus: recensio articuli. Med J Islam Repub Iran. 2013 Nov;
[3] Draper MP, Bhatia B, Assefa H, Honeyman L, Garrity-Ryan LK, Verma AK, Gut J, Larson K, Donatelli J, Macone A, Klausner K, Leahy RG, Odinecs A, Ohemeng K, Rosenthal PJ, Nemesianus ML. In vitro et efficacibus antimalarialibus vivo tetracyclinis optimized. Antimicrob Agentia Chemother. 2013 Iul;
Descriptio
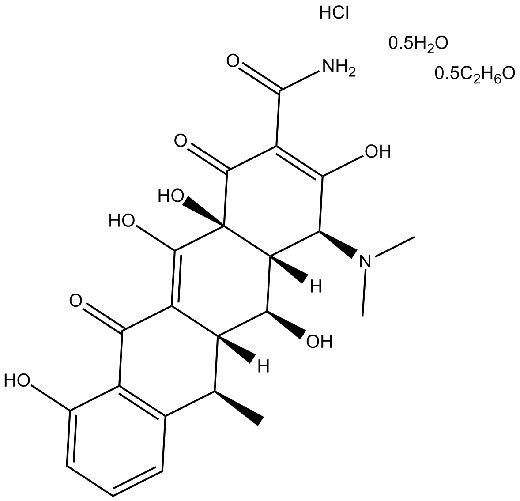
Doxycyclinum (hyclatum) (hemihydratum doxycyclinum hydrochloridum hemiethanolatum), antibioticum, ore activo et lato spectro metalloproteinase (MMP) inhibitor est.
Fusce Trial
| NCT Number | Sponsor | Conditio | Satus Date | Phase |
| NCT00246324 | Louisiana State University Health Sciences Centre Shreveport|Biogen | Multiplex Sclerosis | Decembris 2003 | Phase 4 |
| NCT00910715 | Universitas Medical Centrum Ljubljana | Erythema Chronicum Migrans | Iunii 2009 | Non Lorem |
| NCT00243893 | University of California, San Francisco|National Institute of Neurological Disorders and Stroke (NINDS) | Aneurysms|Arteriovenous Malformationes | Iulii 2004 | Phase 1 |
| NCT00126399 | CollaGenex Pharmaceuticals | Rosacea | Iunii 2004 | Phase 3 |
| NCT01318356 | Radboud University|ZonMw: Organizationis Belgii ad Salutem Research et Development | Q Febris|Fatigue Syndrome, Chronic| | April 2011 | Phase 4 |
| NCT00177333 | Universitas Pittsburgh | Abortus, Adductus|Vomitus | Septembris 2005 | Phase 4 |
| NCT00007735 | US Department of Veterani Negotiis | Pfizer | United States Department Defensionis | VA Office of Research and Development | Persicum Syndrome|Mycoplasma Infectiones | Ianuarii 1999 | Phase 3 |
| NCT00351273 | University of South Florida|National Institute of Arthritis and Musculoskeletal and skin Morbos | Arthritis, Reactive|Reiter Disease | May 2006 | Phase 3 |
| NCT00469261 | Careggi Hospitalis | Myocardial Infarction | Left Ventricular vestibulum molestie lacus | Maii 2007 | Phase 2 |
| NCT00547170 | University of Pittsburgh | Endometritis | Ianuarii 2007 | Phase 4 |
| NCT01475708 | Universitas Medical Centrum Ljubljana | Lyme Borreliosis | May 2011 |
|
| NCT01368341 | Morten Lindbaek|Norwegian Institute of Public Health|Sorlandet Hospital HF|Norwegian University of Life Sciences|University of Osloy. | Erythema Migrans|Erythema Chronicum Migrans|Borreliosis|Lyme Disease|Early Lyme Disease | Iunii 2011 | Phase 4 |
| NCT02538224 | Universitas Azad islamica, Tehran | Chronica Periodontitis | Iulii 2013 | Phase 2|Phase 3 |
| NCT00066027 | University of Nebraska|National Institute of Dental and Craniofacial Research (NIDCR) | Periodontitis | Iunii 2002 | Phase 3 |
| NCT00376493 | Hospitalis de Clinicas de Porto Alegre | Septic Abortus | May 2006 | Phase 4 |
| NCT03448731 | Fundacion CRIS de Investigación para Vencer el Cáncer|Amgen|Apices Soluciones SL | Cutis Toxicity | May 10, 2018 | Phase 2 |
| NCT00989742 | Universitas Nottinghamiae | Lymphangioleiomyomatosis|tuberosis Sclerosis | Iulii 2009 | Phase 4 |
| NCT01438515 | Horizon Health Network | Methicillin-repugnans Staphylococcus Aureus | August 2008 | Non Lorem |
| NCT02929121 | Negotium vis pro Global Health|United States Agency pro International Development (USAID) | Lymphedema|Lymphatica Filariasis|Filariasis | Ianuarii 15, 2019 | Phase 3 |
| NCT00952861 | Odense University Hospital|Kolding Sygehus|Svendborg Hospital|Fredericia Hospital|Naestved Hospital|Hillerod Hospital,Dania|Regio Syddanmark|Danmarks Lungeforening|Danish National Research Foundation | Morbus pulmonalis, Obstructivus chronicus | October 2009 | Phase 4 |
| NCT00138801 | Hospitium Sorlandet HF | Lyma Neuroborreliosis | Martii 2004 | Phase 3 |
| NCT00942006 | Universitas Medical Centrum Ljubljana | Suspecta Early Lyme Neuroborreliosis | Iulii 2009 | Non Lorem |
| NCT02713607 | Universitas Californiae, Davis | Acne Vulgaris | Martii 2016 | Phase 1|Phase 2 |
| NCT00560703 | Galderma | Blepharitis|Meibomianitis|Dry Eye | Novembris 2007 | Phase 2 |
| NCT01014260 | Johns Hopkins University | Morbus cardiovascularis | September 2010 | Phase 4 |
| NCT00000938 | National Institute of Allergy and Infectious Diseases (NIAID) | Lyma Morbus | Phase 3 | |
| NCT01398072 | University College, London|Royal Free Hampstead NHS Trust|University of Cambridge|National Institute for Health Research, United Kingdom | Morbus pulmonalis Obstructivus chronicus (COPD). | December 2011 | Phase 3 |
| NCT03479502 | Vanderbilt University Medical Centre | | Tenaces Capsulitis | Humerum tenaces Capsulitis non specificatae | Humerum gelidum | Ianuarii 5, 2018 | Phase 4 |
| NCT02929134 | Negotium vis pro Global Health|United States Agency pro International Development (USAID) | Lymphedema|Lymphatica Filariasis|Filariasis | Februarii 16, 2018 | Phase 3 |
| NCT00480532 | Oregon Health and Science University | Contraceptiva, Oral | Maii 2007 | Non Lorem |
| NCT01594827 | Johns Hopkins University|Case Western Reserve University|Cystic Fibrosis Foundation | Cystic Fibrosis | October 2012 | Phase 2 |
| NCT01744093 | Weill Collegium Medical University Cornell|National Cordis, Lung, et Institutum Sanguinis (NHLBI) | HIV|Chronic Obstructive Morbus pulmonalis (COPD)|Emphysema | Die 17 mensis Iulii | Non Lorem |
| NCT03530319 | National Taiwan University Hospitalis | Pneumonia, Mycoplasma | die 10 mensis Novembris anno 2018 | Non Lorem |
| NCT04167085 | Universitas Californiae, Los Angeles | Epistaxis | December 18, 2017 | Phase 4 |
| NCT01411202 | Ottawa Hospitalis Research Institute | Malignant Pleurae Effusion | Iunii 2011 | Phase 2 |
| NCT01474590 | Galderma | Acne | November 2011 | Phase 3 |
| NCT00649571 | Mylan Pharmaceuticals | Sanus | Iulii 2005 | Phase 1 |
| NCT02899000 | Galderma Laboratorium, LP | Acne Vulgaris | die 29 mensis Iulii anno 2016 | Phase 4 |
| NCT00538967 | Leiden University Medical Centre | Aorta aneurysma, abdominal | Maii 2002 | Phase 2 |
| NCT00439400 | Alacrity Biosciences, Inc. | Siccus oculus | Februarii 2007 | Phase 2 |
| NCT00917553 | Thomas Gardner|Penn State University|Juvenile Diabetes Research Foundation|Milton S. Hershey Medical Center | Diabetic Retinopathia | Iulii 2009 | Phase 2 |
| NCT00495313 | CollaGenex Pharmaceuticals | Rosacea | Martii 2007 | Phase 4 |
| NCT01855360 | Brigham ac Women's Hospitalis | Amyloidosis; Cordis (Manifestation)|Senile Cardiac Amyloidosis | Iunii 2013 | Phase 1|Phase 2 |
| NCT00419848 | Shahid Beheshti University of Medical Sciences | Acne | August 2006 | Phase 2 |
| NCT03532464 | University Hospital, Bordeaux|USC EA 3671 Infectiones humaines à mycoplasmes et à chlamydiae | Chlamydia Trachomatis Infectio|Vaginal Infection|Anal Infection | Die 1 Iulii 2018 | Phase 4 |
| NCT02756403 | Medstar Health Research Institute|Societatis familiae congue | Primo Trimester Abortus | Martii 2016 | Non Lorem |
| NCT00353158 | National Institute of Arthritis and Musculoskeletal and Cutis Morbis (NIAMS)|National Institutes of Health National Centre (CC) | Sanus voluntariorum | | 11 Iulii 2006 | Phase 1 |
| NCT01317433 | Institut Cancerologie de l´Ouest | Colorectal Cancer Metastatic|Skin Toxicity | December 2010 | Phase 3 |
| NCT01658995 | Petra M. Casey|Mayo Clinic | ESI relatas sanguinem | September 13, 2012 | Phase 3 |
| NCT03968562 | State University of New York - Downstate Medical Center | alvos | May 15, 2019 | Phase 2 |
| NCT02569437 | Icahn Scholam Medicinae in monte Sinai | Polypus Nasi Sinus | September 2014 | Phase 2 |
| NCT01198509 | NYU Langone Health|National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)|Memorial Sloan Kettering Cancer Center | Rheumatoid Arthritis|Psoriatic Arthritis|Periodontal Disease | Ianuarii 2010 | Non Lorem |
| NCT01163994 | Universitas Medical Centrum Ljubljana | Multa Erythema Migrans | June 2010 | Non Lorem |
| NCT02388477 | Milton S. Hershey Medical Center | Rotator Cuff Laesio | Non Lorem | |
| NCT01100295 | Lymphoma Study Group Extranodal Internationalis (IELSG) | Non Hodgkin Lymphoma | Septembris 2006 | Phase 2 |
| NCT00775918 | Ranbaxy Laboratories Limited|Ranbaxy Inc. | Sanus | Iunii 2005 | Non Lorem |
| NCT04050540 | University of Washington|Kenya Medical Research Institute|Kenya National AIDS & STI Control Programme|University of California, San Francisco|National Institute of Allergy and Infectious Diseases (NIAID) | HIV Infection | HIV + AIDS | Neisseria Gonorrheae Infectio | | Februarii 5, 2020 | Phase 4 |
| NCT02562651 | Academia Scientiarum Medicarum | Vascularis Morbis|Cardiovasculares Diseases|Acuti Myocardial Infarctionis | Februarii 2014 | Phase 2|Phase 3 |
| NCT00001101 | National Institute of Allergy and Infectious Diseases (NIAID) | Lyma Morbus | Phase 3 | |
| NCT00340691 | National Institute of Allergy and Infectious Diseases (NIAID)|National Institutes of Health National Center (CC) | Mansonella Perstans Infection|Mp Microfilaremia | die 6 mensis Decembris anno 2004 | Phase 2 |
| NCT01112059 | Universitas Alabama apud Birmingham|Cysticam Fibrosis Foundation | Cystic Fibrosis | November 2008 | Non Lorem |
| NCT00652704 | Par Pharmaceutical, Inc.|Anapharm | Ad determinare Bioequivalence sub H Conditions | Iulii 1999 | Phase 1 |
| NCT01783860 | Tehran University of Sciences Medical | Posterior Blepharitis | Ianuarii 2013 | Phase 2 |
| NCT02564471 | State University of New York - Upstate Medical University|Walter Reed Army Institute of Research (WRAIR)|Kansas State University | Rabies | Aprilis 2016 | Phase 4 |
| NCT04206631 | Universitas Indonesia | Acne Vulgaris | Aprilis 1, 2015 | Phase 1 |
| NCT03956446 | University Medical Centre Ljubljana|University Ljubljana Schola Medicinae, Slovenia | Tick Rome Encephalitis | Septembris 1, 2014 | Non Lorem |
| NCT03960411 | Felix Chikita Fredy, MD|National Cardiovascular Center Harapan Kita Hospital Indonesia|Indonesia University | ST Elevatio Myocardialis Infarctus | May 25, 2019 | Phase 3 |
| NCT00322465 | National Institute of Allergy and Infectious Diseases (NIAID) | Urethritis | Novembris 2006 | Phase 2 |
| NCT01375491 | University of California, San Diego|Ruth L. Kirschstein National Research Service Award|National Institute of Diabetes and Digestive and Renibus Morbis (NIDDK)|National Center for Research Resources (NCRR) | Typus 2 Diabetes|Obesity | October 2009 | Phase 4 |
| NCT03478436 | Universitas medica Viennensis|Dr. Reddy´s Laboratories Press | Rosacea | Iulii 2016 | Phase 1 |
| NCT01207739 | Radboud University|Sint Maartenskliniek|ZonMw: Organizationis Belgii pro Health Research and Development | Lyme Disease|Borrelia Infection | September 2010 | Phase 4 |
| NCT00939562 | Pfizer | Bacteria Infectio | November 2008 | Phase 4 |
| NCT03608774 | National Institute of Allergy and Infectious Diseases (NIAID) | Anal Chlamydia Infection | Die 26 Iunii 2018 | Phase 4 |
| NCT02281643 | Kwame Nkrumah University of Science and Technology|University of Bonn|Heinrich-Heine University, Duesseldorf | Mansonella Perstans Infectio | Buruli Ulceris | Tuberculosis | | October 2014 | Phase 2 |
| NCT00066066 | The Forsyth Institute|National Institute of dental and Craniofacial Research (NIDCR) | Periodontitis|Periodontal Diseases | Iulii 2003 | Phase 2 |
| NCT01798225 | Medical University of South Carolina|National Center for Research Resources (NCRR) | Type 2 Diabetes Mellitus | Decembris 2007 | Phase 4 |
| NCT00612573 | Warner Chilcott | Acne Vulgaris | Februarii 2008 | Phase 2 |
| NCT01631617 | National Institute of Arthritis and Musculoskeletal and Cutis Morbis (NIAMS)|National Institutes of Health National Centre (CC) | Eczema|Dermatitis| Cutis Diseases, Genetic|Dermatitis, Atopic|Skin Morbis | Septembris 18, 2012 | Phase 2 |
| NCT03173053 | Radboud University|ZonMw: Organizationis Belgii pro Health Research and Development | Academisch Medisch Centrum - Universiteit van Amsterdam (AMC-UvA)|Aalborg University Hospital|Rigshospitalet, Denmark. | Staphylococcus Aureus|Motility Disorder | Februarii 8, 2018 | Non Lorem |
| NCT00715858 | McMaster University|Medicis´ Services Incorporated Foundation | Alzheimer´s Morbus | May 2008 | Phase 3 |
| NCT03584919 | Universitas Medical Centrum Ljubljana | Erythema Chronicum Migrans | Die 1 mensis Iunii anno 2006 | Non Lorem |
| NCT01469585 | University of Hawaii|Charles Drew University of Medicine and Science|Meharry Medical College | Breakthrough Cruenta | November 2011 | Non Lorem |
| NCT02759120 | Weill Medical College of Cornell University|Dux Clinical Research Institute|University of Chicago|University of Washington|University of Pittsburgh|National Heart, Lung and Sanguinis Institutum (NHLBI) | Pulmonarium idiopathicum Fibrosis | Martii 22, 2017 | Phase 3 |
| NCT02735837 | Amirhossein Farahmand | | Diabetes Mellitus Cum Morbus periodontal | Ianuarii 2015 | Phase 2|Phase 3 |
| NCT03655197 | Universitas Californiae, Davis | Rosacea|Ocularis Rosacea| | November 2, 2017 | Mane Phase 1 |
| NCT01188954 | Northwell Health | Seroma | Ianuarii 2010 | Non Lorem |
| NCT00388778 | Shahid Beheshti University of Medical Sciences | Acne|Inflammatio | Octobris 2005 | Phase 2|Phase 3 |
| NCT01087476 | Metropolitanus Autonomus Universitatis | Instituto Nacional de Cancerologia de Mexico | Mucositis | May 2010 | Phase 2 |
| NCT02174757 | CD Pharma India Pvt. Ltd.|Sree Mookambika Institutum Scientiarum dentalium | Chronica Periodontitis | August 2014 | Phase 3 |
| NCT03911440 | National Taiwan University Hospitalis | Pneumonia atypical | die 10 mensis Novembris anno 2018 | Non Lorem |
| NCT02553083 | Rabin Medical Centre | Bacterial Infectio Ob Helicobacter Pylori (H. Pylori) | October 22, 2015 | Phase 4 |
| NCT04234945 | Ahmadu Bello University Teaching Hospital | Infecunditas, Female|Pelvicium Inflammatoriae Morbus | Ianuarii 13, 2020 | Non Lorem |
| NCT00892281 | Galderma Laboratorium, LP | Rosacea | Aprilis 2009 | Phase 4 |
| NCT02913118 | Qingfeng Pharmaceutical Group | Pneumonia? | Iulii 2016 | Phase 4 |
| NCT04153604 | Methodist Health System | Cirrhosis|Spontaneous Bacterial Peritonitis | November 4, 2019 |
|
| NCT03153267 | University Medical Centre Ljubljana|University Ljubljana Schola Medicinae, Slovenia | Erythema Chronicum Migrans | Iunii 1, 2017 | Non Lorem |
| NCT03116659 | James J. Peters Veterans Negotiis Medical Centre | Lymphoma, T-Cella, Cutaneous | Februarii 1, 2018 | Mane Phase 1 |
| NCT03401372 | Jian Li|Peking University Primum Hospitalis Chinese PLA Hospitalis Generalis Beijing Chao Yang Hospital|Occidentalis Sinis Hospitalis Socius Sichuan University|Tongji Hospitalis Socius Tongji Medical College of HUST|Unionis Hospitalis Socius Tongji Medical College of HUST|Shanghai Changzheng Hospital| Nanfang Hospitium Southern Medical University|Peking Unionis Medical College Hospital | Amyloidosis; Systemic | Aprilis 21, 2018 | Non Lorem |
| NCT01380496 | Par Pharmaceutical, Inc.|Anapharm | Ad determinare Bioequivalence sub H Conditions | Novembris 1999 | Phase 1 |
| NCT03083197 | University of Oxford|Shoklo Malaria Research Unit|Chiangrai Prachanukroh Hospital | LIQUET Typhus | October 15, 2017 | Phase 4 |
| NCT00237016 | Medical Corps, Israel Defensionis Force | Relapsus Febris, Tick-Borne|Jarisch Herxheimer Reaction | Aprilis MMII | Phase 2|Phase 3 |
| NCT01308619 | Galderma Laboratorium, LP | Rosacea | April 2011 | Phase 4 |
| NCT01198912 | Hospitalis Universitatis Gandavensis | Chronic Rhinosinusitis|Nasal Polyps | die 22 mensis Novembris anno 2011 | Phase 2 |
| NCT02016365 | Umeå Universitas | Transthyretin Amyloidosis|Cardiomyopathy | Februarii 2012 | Phase 2 |
| NCT00783523 | Universitas Californiae, San Francisco | Arteriovenous Malformationes|Cavernous Angiomas|Bin Aneurysms | Martii 2008 | Phase 1 |
| NCT03337932 | Universitas Medical Centrum Ljubljana | Erythema Chronicum Migrans | Ianuarii 1, 2018 | Non Lorem |
| NCT00568711 | Dong-Min Kim|Chosun University Hospital | LIQUET Typhus | Septembris 2006 | Non Lorem |
| NCT01874860 | University of Louisville|James Graham Brown Cancer Center | Colorectal Cancer | | August 2013 | Phase 2 |
| NCT01171859 | IRCCS Policlinico S. Matteo | Transthyretin Amyloidosis | July 2010 | Phase 2 |
| NCT01653522 | Clinic Cleveland | Migraine Disorders|Headache, Migraine|Migraine|Migraine Headache|Migraine With Aura|Migraine Without Aura|Headache Disorders, Primary | Iulii 2012 | Non Lorem |
| NCT01200910 | Lymphoma Study Group Extranodal Internationalis (IELSG) | Marginal Zone Lymphoma Ocularis Adnexal | Martii 2013 | Phase 2 |
| NCT01323101 | Universitas Southern California | Cystic Fibrosis | Aprilis 2008 | Phase 4 |
| NCT00829764 | USA Teva Pharmaceuticals | Sanus | Octobris 2006 | Phase 1 |
| NCT01668498 | AIO-Studien-gGmbH | Ras-wildtype Cancer Colorectal | May 2011 | Phase 2 |
| NCT01030666 | Peter Eickholz|Heidelberg University|Dr. August Wolff GmbH & Co. KG Arzneimittel|Gaba International AG|Goethe University | Periodontitis | Aprilis 2007 | Phase 4 |
| NCT00012688 | US Department of Veterani Negotiis|Colgate-Periogard-Dentsply|VA Office of Research and Development | Diabetes Mellitus|Pauper Glycemic Control|Peridontal Disease | Non Lorem | |
| NCT01885910 | Derm Research, PLLC|WFH MEDICAL, LLC | Acne Vulgaris | Iulii 2013 | Phase 4 |
| NCT02328469 | University Medical Centre Ljubljana | | Aseptic Meningitis | Iunii 2014 |
|
| NCT00355602 | University of Dunde|Tenovus Scotland | Colitis, Ulcerativum | Iulii 2006 | Non Lorem |
| NCT02006032 | Hamilton Health Sciences Corporation|Hamilton Academic Health Sciences Organization | Rectum | May 2016 | Phase 2 |
| NCT01465802 | Pfizer | Non Small Cell Lung Cancer (NSCLC) | December 26, 2011 | Phase 2 |
| NCT02623959 | MD Anderson Cancer Center | Provectus Cancer|Malignant Pleurae Effusions | Die 27 Aprilis, 2016 | Phase 4 |
| NCT03481972 | IRCCS Policlinico S. Matteo | TTR Cardiac Amyloidosis | April 11, 2018 | Phase 3 |
| NCT00428818 | Universitas Texas Southwestern Medical Center | Infectio | August 2005 | Non Lorem |
| NCT01355622 | Virginia Reipublicae Universitatis | Cordis Systolici Defectum (NYHA II-III) | Iulii 2012 | Phase 2 |
| NCT01886560 | Sol Universitatis Yat-sen | Oculus Ardens | September 2013 | Phase 2|Phase 3 |
| NCT04239755 | Damanhour University|Tanta University | Laesio traumatica cerebrum | December 15, 2019 | Phase 4 |
| NCT02204254 | Centrum Hospitalier Universitaire de Nice | Rosacea | Martii 2014 | Non Lorem |
| NCT00837213 | Stiefel, a GSK Company|GlaxoSmithKline | Acne | August 2007 | Phase 4 |
| NCT03115177 | Rush University Medical Center | Osteoarthritis | November 2015 | Non Lorem |
| NCT03618108 | Cadrock Pty | Coronarium Cor Morbum|Chlamydophila Pneumoniae Infectiones | Aprilis 4, 2018 | Phase 2 |
| NCT03435952 | MD Anderson Cancer Center|BioMed Valley Discoveries, Inc|Merck Sharp & Dohme Corp. | malignum Neoplasma Pectus|Malignum Neoplasmatum digestivorum Organorum|malignum Neoplasmatum oculi cerebri et partium nervorum centralium | malignorum organorum femininorum genitalium malignorum Sites|Malignant Neoplasmata labii oralis cavitatis et pharyngis | maligni Neoplasmata organorum genitalium masculinorum | malignorum Neoplasmatum mesothelialium et mollium textorum | malignum neoplasmatum respiratorii et organorum intrathoracicorum | malignum neoplasmatum thyroideum et alia glandularum endocrinarum | Die 10 iulii 2018 | Phase 1 |
| NCT01867294 | Academic and Community Cancer Research United|National Cancer Institute (NCI) | Provectus malignum Neoplasma|Dermatologic Complicationem | August 31, 2012 | Phase 2 |
| NCT01677286 | Boston University | Amyloidosis | Iulii 2012 | Phase 2 |
| NCT00511875 | Thomas Gardner|Juvenile Diabetes Research Foundation|Milton S. Hershey Medical Center | Diabetic Retinopathia | Iulii 2008 | Phase 2 |
| NCT04108897 | Johns Hopkins University | Rosacea | September 17, 2019 | Mane Phase 1 |
| NCT00631501 | Kaunas University of Medicine, University Hospital, Linkoeping | Epicondylalgia lateralis (Tennis Cubitus) | Non Lorem | |
| NCT02203682 | Sol Universitatis Yat-sen | Gravia Ophthalmopathia | Gravia Morbus | Oculi Morborum | Thyroideum Morborum | Iulii 2014 | Phase 2 |
| NCT0205653 | Consilium Indicum de Investigatione Medica | Filarial; Infestation | Februarii 2009 | Phase 4 |
| NCT03585140 | Centro Dermatologico Ladislao de la Pascua Dr | Acne Vulgaris|Diet Modification | Ianuarii 1, 2016 | Non Lorem |
| NCT02147262 | University Medical Centre Ljubljana|University of Ljubljana Schola Medicinae, Slovenia | | Chronica Atrophic Acrodermatitis | Iulii 2013 | Non Lorem |
| NCT02220751 | Universitas Sao Paulo|Fundaça de Amparo à Pesquisa do Estado de São Paulo | Periodontitis|Type 2 Diabetes Mellitus | Martius 2009 | Phase 3 |
| NCT01825408 | Universitas Carolinae Septentrionalis, Capella Hill | Sinusitis | Februarii 2013 | Phase 4 |
| NCT02884713 | Rex Hospitalis Specialist Faisal & Research Centre | GASTRITIS | Iunii 2013 | Non Lorem |
| NCT02726646 | University of Campinas, Brazil|Pontificia Universidade Catolica de Sao Paulo | Chronica Periodontitis | Iunii 2015 | Phase 2 |
| NCT00883818 | Samsung Center | Vesica nimis | Ianuarii 2007 | Phase 4 |
| NCT00829790 | USA Teva Pharmaceuticals | Sanus | Octobris 2006 | Phase 1 |
| NCT01949233 | University of Oxford|Oxford University Hospitals NHS Trust | Marfan Syndrome | October 2013 | Phase 2 |
| NCT01158192 | University Medical Center Ljubljana|Slovenian Research Agency | Erythema Migrans|Post-lyme Morbus Symptomata | Iunii 2006 | Phase 4 |
| NCT02845024 | Universitas Azad islamica, Tehran | Diabetes Mellitus Cum Morbus periodontal | September 2014 | Non Lorem |
| NCT01879930 | University Hospitalis Inselspital, Berne | Chronica Pelvici Dolor Syndrome | | November 2012 | Phase 4 |
| NCT00041977 | CollaGenex Pharmaceuticals | Acne Rosacea | Iunii 2002 | Phase 3 |
| NCT02341209 | Roffensis Hospitalis Generalis | Cutaneous T-cell Lymphoma|Mycosis Fungoides|Sezary Syndrome | February 6, 2018 | Phase 2 |
| NCT00002872 | Eastern Cooperative Oncology Group|National Cancer Institute (NCI)|North Central Cancer Treatment Group | Cancer metastatic | Novembris 1996 | Phase 3 |
| NCT03162497 | Universitas medica Viennensis | Oculus siccus Syndromes|Meibomian Gland Dysfunction | Ianuarii 8, 2018 | Phase 4 |
| NCT01418742 | Gesellschaft fur Medizinische Innovation? Hamatologie und Onkologie mbH|ClinAssess GmbH | Colorectal Carcinoma | August 2011 | Phase 2 |
| NCT00980148 | National Institute of Allergy and Infectious Diseases (NIAID) | Chlamydial Infectio | December 2009 | Phase 3 |
| NCT03342456 | Tertium Xiangya Hospitalis Centralis Meridionalis University|Livzon Pharmaceutical Group Inc.|Yung Shin Pharm. Ind. Co., Ltd. | Duodenal Ulcer Ob Helicobacter Pylori | December 13, 2017 | Phase 4 |
| NCT04310930 | University of Queensland|Australian Department of Health|Fibrosi Hospitalis Liberi Fundationis|Cysticae Fibrosis Foundation|Newcastle University|Griffith University|Erasmus Medical Centre|Monash University|University of Copenhagen|Hôpital Cochin|South Australian Health and Medical Research Institute|University. James Cook University, Queensland, Australia|Murdoch Childrens Research Institute | Pulmonalis Morbus Ob Mycobacteria (Diagnosis) | Martii 2020 | Phase 2|Phase 3 |
| NCT03709459 | Kirby Institute|South Australian Health and Medical Research Institute|Monash University | STIs praeventionis | December 17, 2019 |
|
| NCT04067011 | BioSolutions Emergens | Lorem Advanced Research and Development Authority | Anthrax | August 12, 2019 | Phase 2 |
| NCT02844634 | British Columbia Centrum pro Morbus Imperium | HIV|Syphilis | May 15, 2018 | Phase 4 |
| NCT00647959 | Mylan Pharmaceuticals | Sanus | Martii 2006 | Phase 1 |
| NCT00170222 | Medical Centre Alkmaar | Morbus pulmonalis obstructivus chronicus | Iulii 2002 | Phase 4 |
| NCT03075891 | Galderma | Rosacea | Die 5 Iulii 2017 | Phase 4 |
| NCT00031499 | National Institute of Allergy and Infectious Diseases (NIAID) | Syphilis | Iunii 2000 | Phase 3 |
| NCT01205464 | Linkoeping University | Fatigue|Radicular Pain|Cognitive Dysfunction|Paresthesia|Paresis | Februarii 2005 | Non Lorem |
| NCT01301586 | Nexgen Dermatologics, Inc. | ACNE VULGARIS | November 2010 | Phase 1|Phase 2 |
| NCT02305940 | Imperial College London | Morbus pulmonalis Obstructivus chronicus (COPD) | Iulii 2014 | Phase 3 |
| NCT00351182 | Dong-Min Kim|Chosun University Hospital | LIQUET Typhus | Septembris 2005 | Phase 3 |
| NCT03334682 | Nantes University Hospitalis | Acne Vulgaris | January 31, 2018 | Phase 3 |
| NCT01788215 | Universitas Roffensis | Polycystic Ovarian Syndrome (PCOS) | November 2010 | Phase 3 |
| NCT03076281 | Sidney Kimmel Cancer Centre apud Thomas Jefferson University| | Larynx|LIP|Oral Cavity|Pharynx | Aprilis 3, 2017 | Phase 2 |
| NCT00439166 | Hamilton Health Sciences Corporation|The Physicians' Services Incorporated Foundation|McMaster University | Alzheimer´s Morbus | Februarii 2007 | Phase 3 |
| NCT02463942 | University Medical Centre Ljubljana|University Ljubljana Schola Medicinae, Slovenia | Tick-fertur Encephalitis | September 2014 | Non Lorem |
| NCT00803842 | Universitas Northwestern | Non Small Cell Lung Cancer | October 2008 | Non Lorem |
| NCT02086591 | Universitas Roffensis | Adult Diffuse Large B-Cell Lymphoma|Mantle Cell Lymphoma Recurrente| Lymphoma, Follicular| Marginal Zone B-Cell Lymphoma|Malignum Lymphoma - Lymphoplasmacytic|Waldenstrom Macroglobulinemia|Lymphoma Lymphocytic Lymphoma|Chronic Lymphocytic Leukemia (CLL)| | Martii 2014 | Phase 2 |
| NCT03980223 | University of California, San Francisco|University of Washington|National Institute of Allergy and Infectious Diseases (NIAID)|Mayne Pharma International Pty Ltd|San Francisco Department of Public Health | Gonorrhoea|Chlamydia|Syphilis | November 26, 2019 | Phase 4 |
| NCT00355459 | Universitas Texas Southwestern Medical Center | Siccus oculus Syndrome | August 2005 | Non Lorem |
| NCT01254799 | Omar Mamdouh Shaaban|Assiut University | Haemorrhagia uterina | Ianuarii 2008 | Phase 3 |
| NCT01547325 | NanoSHIFT LLC|United States Department of Defence | Vulnera chirurgicam dehisced | May 2012 | Non Lorem |
| NCT00653380 | Par Pharmaceutical, Inc.|Anapharm | Ad determinare Bioequivalence sub jejuniis | Septembris MCMXCIX | Phase 1 |
| NCT00635609 | Warner Chilcott | Acne Vulgaris | Martii 2008 | Phase 4 |
| NCT03765931 | Institut de Recherche pour le Developpement | Febris | Iulii 2016 | Phase 4 |
| NCT01160640 | Harold Wiesenfeld|National Institute of Allergy and Infectious Diseases (NIAID)|University of Pittsburgh | Inflammatio morbi pelvis | November 2010 | Phase 2 |
| NCT01756833 | University of Maryland, Baltimore|National Institute on Aging (NIA) | Aneurysmate | May 2013 | Phase 2 |
| NCT00688064 | Galderma | Gravis Acne Vulgaris | August 2008 | Phase 3 |
| NCT01320033 | Galderma | Acne Vulgaris | die 29 mensis Martii anno 2011 | Phase 2 |
| NCT03397004 | Hospitalis Sancti Michaelis, Toronto|Barrow Neurological Institute|Dux University|Feinstein Institute for Medical Research|University of Pittsburgh|Sunnybrook Health Sciences Center | Hereditarius haemorrhagic Telangiectasia (HHT) | September 12, 2018 | Phase 2 |
| NCT01635530 | Turku University Hospitalis | Lyma Neuroborreliosis | August 2012 | Phase 4 |
| NCT03727620 | Mohammed V Souissi University | petulantia Periodontitis | die 6 mensis Ianuarii anno 2014 | Phase 1|Phase 2 |
| NCT02688738 | Rothman Institutum Orthopedicum | Propionibacterium | Martii 2015 | Non Lorem |
| NCT00358462 | University of Washington|National Institute of Allergy and Infectious Diseases (NIAID) | Urethritis | Ianuarii 2007 | Phase 3 |
| NCT02864550 | British Columbia Centrum pro Morbus Imperium | Syphilis | | August 15, 2019 | Phase 4 |
| NCT01595594 | Universitas Sao Paulo|Fundaça de Amparo à Pesquisa do Estado de São Paulo | Morbus periodontal|Type 2 Diabetes | Martii 2010 | Phase 3 |
| NCT00964834 | PharmAthene, Inc. | Anthrax | Iulii 2009 | Phase 1 |
| NCT01809444 | Sol Universitatis Yat-sen | Thyroideum Associated Opthalmopathies | November 2012 | Phase 2|Phase 3 |
| NCT01590082 | MD Anderson Cancer Centre|National Institutes of Health (NIH)|National Cancer Institute (NCI) | Melanoma | November 2012 | Phase 1|Phase 2 |
| NCT00207584 | Centra Morbus Imperium ac Praeventionis | Mycoplasma Pneumoniae | Ianuarii 1994 | Non Lorem |
| NCT00775177 | Ranbaxy Laboratories Limited|Ranbaxy Inc. | Sanus | Iunii 2005 | Non Lorem |
| NCT03462329 | Universitas Medical Centrum Ljubljana | Erythema Migrans | Iunii 1, 2018 | Non Lorem |
| NCT00000403 | Indiana University|National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)|National Institute on Aging (NIA) | Osteoarthritis | Septembris 1996 | Phase 3 |
| NCT03508232 | University of Alberta|Regia Alexandra Hospital | ST Segmentum Elevatio Myocardial Infarctio|Cordis Defectum | Die 6 mensis Ianuarii, anno 2020 | Phase 2 |
| NCT02553473 | Hospitium Sorlandet HF | Neuroborreliosis, Borrelia Burgdorferi | October 2015 | Phase 3 |
| NCT02207556 | Collegium Medicum Wisconsin | Systemic amyloidosis primaria | Octobris 1, 2014 | Phase 2 |
| NCT01783106 | Royal Liverpool University Hospital|National Association for Colitis and Crohn´s Disease|National Institute for Health Research, United Kingdom | Crohn´s Morbus | Februarii 1, 2014 | Phase 2 |
| NCT00353743 | Hospitalis de Clinicas de Porto Alegre | Abortus, Septic | May 2006 | Non Lorem |
| NCT0172797 | Sol Universitatis Yat-sen | Gravia Ophthalmopathia | Gravia Morbus | Oculi Morborum | Thyroideum Morborum | October 2012 | Phase 1|Phase 2 |
| NCT00857038 | Alkmaar Medical Centre|Leiden University Medical Centre|University Amstelodami | Inflammatio pulmonis Emphysema | Aprilis 2009 | Phase 4 |
| NCT02774993 | National University Hospital, Singapore|Tan Tock Seng Hospital|National University, Singapore|A*Stella | Tuberculosis | September 2015 | Phase 2 |
| NCT03474458 | IRCCS Policlinico S. Matteo | Cardiac AL Amyloidosis | Februarii 11, 2019 | Phase 2|Phase 3 |
| NCT02874430 | Sidney Kimmel Cancer Centre apud Thomas Jefferson University| | Pectus Carcinoma|Endometrial Clear Cell Adenocarcinoma|Endometrial Serous Adenocarcinoma|Uterine Corpus Cancer|Uterinum Corpus Carcinosarcoma | Die 8 Iunii 2016 | Phase 2 |
| NCT0001685 | University of Michigan|National Institute of Dental and Craniofacial Research (NIDCR) | Diabetes Mellitus, Type 2 | Die 17 Octobris 2001 | Phase 2 |
| NCT00064766 | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) | Endometrial Bleeding|Periodontal Disease | Februarii anno 2003 | Phase 4 |
| NCT00803452 | Universitas Louisville | Blepharitis | Iulii 2008 | Phase 4 |
| NCT01434173 | Bayer|RTI Health Solutions | Medicamento adductus iecoris iniuriam | Iulii 2001 |
|
| NCT00126204 | Barnesia hospitalis Iudaeorum | Aorta aneurysmate | Martii 2004 | Non Lorem |
| NCT01917721 | Hawaii Pacific Health | Kawasaki Morbus | | October 2013 | Phase 2 |
| NCT02775695 | Collegium Medicum Wisconsin | Pancreaticum cancer | Aprilis 3, 2017 | Phase 2 |
| NCT03824340 | Aljazeera Hospitalis | infecunditas | Ianuarii 30, 2019 | Non Lorem |
| NCT01847976 | Ottawa Hospital Research Institute|Canadian Pectus Cancer Foundation | Dolor | August 2013 | Phase 2 |
| NCT02850913 | Makerere University|University of Oxford | Invasiones | Septembris 5, 2016 | Phase 2 |
| NCT00764361 | NanoSHIFT LLC | Ulceris pedis diabetic | Ianuarii 2009 | Phase 2 |
| NCT02036528 | Royer Biomedical, Inc. | Ulceribus diabetic pedis | Ianuarii 2014 | Phase 1|Phase 2 |
| NCT01661985 | Concilium comitatus Ostergotland, Sweden|Statuens Serum Institut | Urethritis|Cervicitis|Genital Mycoplasma Infectio|Chlamydia Trachomatis | Februarii 2010 | Phase 4 |
| NCT01380483 | Par Pharmaceutical, Inc.|Anapharm | Ad determinare Bioequivalence sub jejuniis | Ianuarii 2000 | Phase 1 |
| NCT00648180 | Mylan Pharmaceuticals | Sanus | Iulii 2005 | Phase 1 |
| NCT01426269 | Galderma Laboratorium, LP | Rosacea | September 2011 | Phase 4 |
| NCT02753426 | Universitas Californiae, San Francisco | Chronica Renum Morbus|Cardiorenal Syndrome | Aprilis 2016 | Phase 1 |
| NCT02583282 | Postgraduatum Institutum Medical Educationis et Investigationis | Malignant Pleurae Effusion | August 1, 2015 | Non Lorem |
| NCT02927496 | Negotium vis pro Global Health|United States Agency pro International Development (USAID) | Lymphedema|Lymphatica Filariasis|Filariasis | June 19, 2018 | Phase 3 |
| NCT00652795 | Par Pharmaceutical, Inc.|Anapharm | Ad determinare Bioequivalence sub jejuniis | Iulii 2004 | Phase 1 |
| NCT03956212 | University Medical Centre Ljubljana|University Ljubljana Schola Medicinae, Slovenia | Erythema Migrans | Iunii 1, 2017 | Non Lorem |
| NCT00855595 | Bayer | Papulopustularis Rosacea | Februarii 2009 | Phase 4 |
| NCT03457636 | Derm Research, PLLC | Acne | Martii 19, 2018 | Phase 4 |
| NCT02894268 | Domine vade Shaw hospitalis | Helicobacter Pylori Infection | Februarii 2016 | Phase 4 |
| NCT03465774 | MD Anderson Cancer Centre|National Cancer Institute (NCI) | Malignant Pleurae Effusion | Die 8 Martii 2018 | Mane Phase 1 |
Chemical structure





Rogatio18Qualitas Constantia Aestimatio inceptis quae probaverunt4et6incepta approbantes.

Provectus qualitas internationalis administrationis systematis solidum fundamentum in venditionibus posuit.

Qualitas vigilantia percurrit totum vitae cyclum producti, ut qualitatem et therapeuticum effectum curet.

Negotiis professionales regulares turmas sustinet qualitatem postulatorum in applicatione et adnotatione.


Korea Countec Bottled Packaging linea


Taiwan CVC Bottled Packaging Line


Italia CAM Board Packaging Line

German Fette Compacting Machine

Iaponia Viswill Traba Detector

DCS Control Room







