Rivaroxaban
Background
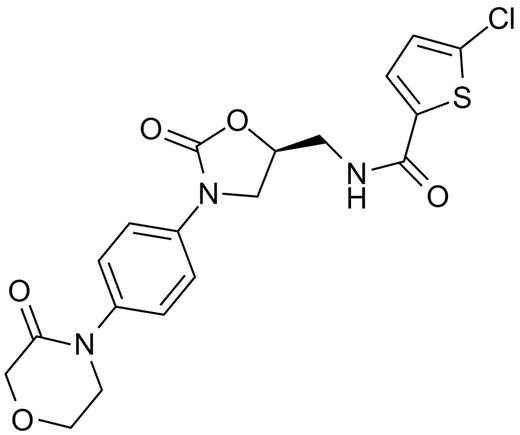
Rivaroxaban, 5-chloro-N-[[(5S)-2-oxo-3-[4-(3-oxomorpholin-4-yl)phenyl]-1,3-oxazolidin-5-yl]methyl]thiophene-2 -carboxamide, est potentia parva inhibitor-moleculi factoris Xa, quae est factor coagulationis in junctura critica in via coagulationis sanguinis proveniens in generatione thrombini et formationis. concretus. Rivaroxaban obligat ad Tyr288 in S1 sinum factoris Xa per commercium Tyr288 et substituentem chlorothiophene medietatem. Inhibitio convertitur (koff = 5x10-3s-1), celeri (kon = 1.7x107 mol/L-1 s-1), et in modum concentrationis-dependens (Ki = 0.4 nmol/L). Rivaroxaban nunc studetur pro curatione VTE, ne eventus cardiovasculares aegros cum syndrome coronaria acuta, ne plaga in patientibus fibrillationis atrialis.
Reference
Elisabeth Perzborn, Susanna Roehrig, Alexander Straub, Dagmar Kubitza, Wolfgang Mueck, et Volker Laux. Rivaroxaban: novus factor oralis Xa inhibitor. Arterioscler Thromb Vasc Biol 2010; 30(3): 376-381
Descriptio
Rivaroxaban (BAV 59-7939) est valde potens.selectivam et directam Factor Xa (FXa) inhibitor, assequendum vehementem quaestum in potentia anti-FXa (IC50 0.7 nM; Ki 0.4 nM).
in Vitro
Rivaroxaban (BAV 59-7939) est oralis, directa Factor Xa (FXa) inhibitor in evolutione ad praecavendam et tractationem thrombosis arteriarum et venarum. Rivaroxaban certatim hominum FXa (Ki 0.4 nM) vetat cum >10 000 duplicium selectivam maiorem quam pro aliis serinis proteasibus; vetat etiam actionem prothrombinasem (IC50 2.1 nM). Rivaroxaban vetat endogenous FXa potentius in homine et lepus plasma (IC50 21 nM) quam rat plasma (IC50 290 nM). Demonstrat effectus anticoagulantes in plasmate humano, prothrombin duplicando tempus (PT) et tempus partialem thromboplastin in 0.23 et 0.69 actuat.μM, respectively.
Rivaroxaban (BAV 59-7939) est potens et selectiva, FXa inhibitor directa cum excellenti in actu vivo ac bonae bioavailability oralis. Rivaroxaban (BAV 59-7939), administratum per bolum iv ante thrombum inducendum, reducit thrombum formationis (ED50 0.1 mg/kg), vetat FXa, et prorogat PT dosem dependentem. PT et FXa leviter afficiuntur in ED50 (1.8 duplici augmento et 32% inhibitionis respective). Ad 0.3 mg/kg (dosis ducens ad inhibitionem thrombi formationis fere completam), Rivaroxaban modice PT prorogat (3.2±0,5-ovili) et actio FXa nia (65±III%).
Repono
| Pulvis | -20°C | III annos |
| 4°C | II annis | |
| In solvendo | -80°C | VI menses |
| -20°C | I mensis |
Chemical structure





Rogatio18Qualitas Constantia Aestimatio inceptis quae probaverunt4et6incepta approbantes.

Provectus qualitas internationalis administrationis systematis solidum fundamentum in venditionibus posuit.

Qualitas vigilantia percurrit totum vitae cyclum producti, ut qualitatem et therapeuticum effectum curet.

Negotiis professionales regulares turmas sustinet qualitatem postulatorum in applicatione et adnotatione.


Korea Countec Bottled Packaging linea


Taiwan CVC Bottled Packaging Line


Italia CAM Board Packaging Line

German Fette Compacting Machine

Iaponia Viswill Traba Detector

DCS Control Room





