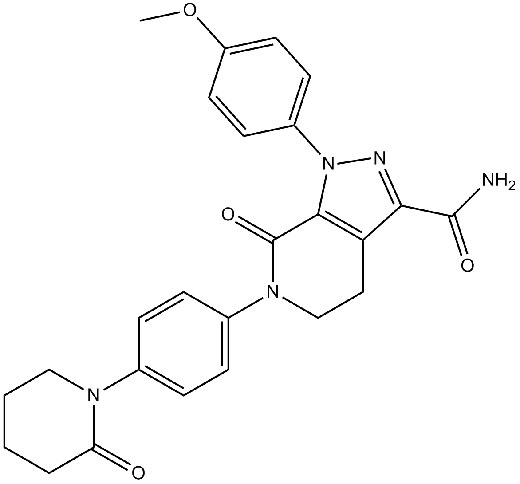
Apixaban
Background
Apixaban inhibitor factoris X admodum selectivus ac convertitur cum Ki valoribus 0,08 nM et 0.17 nM in homine et lepore, respective[1].
Factor X, etiam per eponym Stuart-Prower notum factor, est enzyme cascades coagulationis. Factor X per hydrolysim in factorem X ab utroque facto IX reducitur. Factor Xa est forma reducitur coagulationis factorthrombokinase. Inhibiting Factor Xa offerre potest methodum alternam pro anticoagulation. Direct Xa inhibitores sunt populares anticoagulantes [2].
In vitro: Apixabanhas eminentiam ostendit potentiae, selectivity et efficaciae in Factor Xa cum Ki 0.08 nM et 0.17 nM pro Humano Factor Xa et Rabbit Factor X, respective [1]. Apixaban protrahebat concretionem temporum normalium plasmatis humani cum concentratione (EC2x) 3.6, 0.37, 7.4 et 0.4 µM, quae respective ad tempus prothrombin duplicatum (PT), tempus prothrombin modificatum (mPT), tempus partiale modificatum (mPT), reducitur ad tempus thromboplastin. APTT) et HepTest. Praeterea Apixaban in plasmate leporis et humano summam potentiam ostendit, sed minus in rat et cane plasma in PT et APTT pertentat [III].
In vivo: Apixaban excellentes pharmacokineticas alvi demissiores exhibuit (Cl: 0.02 L kg-1h-1), et volumen humile distributionis (Vdss: 0.2 L/kg) in cane. Praeterea Apixaban etiam mediocrem vitam dimidiam cum T1/2 de 5.8 horis ostendit et bioavailability oris bonam (F: 58%) [1]. In thrombosis arteriovenoso-shunto (AVST), thrombosis venosis (VT) et electrically mediata carotidis arteriarum thrombosis (ECAT) leporis exempla, Apixaban produxit effectus antithromboticos cum EC50 270 nM, 110 nM et 70 nM in modo dose-dependens. ]. Apixaban factor Xa activitatem cum IC50 0.22 μM in lepore ex vivo signanter inhibuit[4]. In sphingas, Apixaban etiam parvum volumen distributionis ostendit (Vdss: 0.17 L kg-1), humilem alvi systemicam (cl: 0.018 L kg-1h-1), et bonam oralem bioavailbilitatem (F: 59%) [5].
Notae:
Pinto DJP, Orwat MJ, Koch S, et al. Inventio 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl) -4, 5, 6, 7-tetrahydro-1 H-pyrazolo [3, 4- c] pyridine-3-carboxamide (Apixaban, BMS-562247), valde potentem, selectivam, efficacem, et viva voce inhibitoris coagulationis sanguinis factoris Xa[J]. Acta chemiae medicinalis, 2007, 50(22): 5339-5356.
Sidhu P S. Direct Factor Xa Inhibitores ut Anticoagulantes[J].
Wong PC, Crain EJ, Xin B, et al. Apixaban, oralis, directa et valde selectiva factor Xa inhibitor: in vitro, antithrombotica et antihemostaticstudia[J]. Acta Thrombosis et Haemostasis, 2008, 6(5): 820-829.
Zhang D, Ipse K, Raghavan N, et al. Metabolismus, pharmacokinetica et pharmacodynamica factoris Xa inhibitoris apixaban in leporibus[J]. Acta thrombosis et thrombolysis, 2010, 29(1): 70-80.
Ipse K, Luettgen JM, Zhang D, et al. Preclinical pharmacokinetica et pharmacodynamica apixaban, factor potens et selectivus Xa inhibitor [J]. Acta medicamentorum metabolismi et pharmacokineticorum Europaeorum, 2011, 36(3): 129-139.
Apixaban inhibitor factoris X admodum selectivus ac convertitur cum Ki valoribus 0,08 nM et 0.17 nM in homine et lepore, respective[1].
Factor X, etiam per eponym Stuart-Prower notum factor, est enzyme cascades coagulationis. Factor X per hydrolysim in factorem X ab utroque facto IX reducitur. Factor Xa est forma reducitur coagulationis factorthrombokinase. Inhibiting Factor Xa offerre potest methodum alternam pro anticoagulation. Direct Xa inhibitores sunt populares anticoagulantes [2].
In vitro: Apixabanhas eminentiam ostendit potentiae, selectivity et efficaciae in Factor Xa cum Ki 0.08 nM et 0.17 nM pro Humano Factor Xa et Rabbit Factor X, respective [1]. Apixaban protrahebat concretionem temporum normalium plasmatis humani cum concentratione (EC2x) 3.6, 0.37, 7.4 et 0.4 µM, quae respective ad tempus prothrombin duplicatum (PT), tempus prothrombin modificatum (mPT), tempus partiale modificatum (mPT), reducitur ad tempus thromboplastin. APTT) et HepTest. Praeterea Apixaban in plasmate leporis et humano summam potentiam ostendit, sed minus in rat et cane plasma in PT et APTT pertentat [III].
In vivo: Apixaban excellentes pharmacokineticas alvi demissiores exhibuit (Cl: 0.02 L kg-1h-1), et volumen humile distributionis (Vdss: 0.2 L/kg) in cane. Praeterea Apixaban etiam mediocrem vitam dimidiam cum T1/2 de 5.8 horis ostendit et bioavailability oris bonam (F: 58%) [1]. In thrombosis arteriovenoso-shunto (AVST), thrombosis venosis (VT) et electrically mediata carotidis arteriarum thrombosis (ECAT) leporis exempla, Apixaban produxit effectus antithromboticos cum EC50 270 nM, 110 nM et 70 nM in modo dose-dependens. ]. Apixaban factor Xa activitatem cum IC50 0.22 μM in lepore ex vivo signanter inhibuit[4]. In sphingas, Apixaban etiam parvum volumen distributionis ostendit (Vdss: 0.17 L kg-1), humilem alvi systemicam (cl: 0.018 L kg-1h-1), et bonam oralem bioavailbilitatem (F: 59%) [5].
Notae:
Pinto DJP, Orwat MJ, Koch S, et al. Inventio 1-(4-methoxyphenyl)-7-oxo-6-(4-(2-oxopiperidin-1-yl) -4, 5, 6, 7-tetrahydro-1 H-pyrazolo [3, 4- c] pyridine-3-carboxamide (Apixaban, BMS-562247), valde potentem, selectivam, efficacem, et viva voce inhibitoris coagulationis sanguinis factoris Xa[J]. Acta chemiae medicinalis, 2007, 50(22): 5339-5356.
Sidhu P S. Direct Factor Xa Inhibitores ut Anticoagulantes[J].
Wong PC, Crain EJ, Xin B, et al. Apixaban, oralis, directa et valde selectiva factor Xa inhibitor: in vitro, antithrombotica et antihemostaticstudia[J]. Acta Thrombosis et Haemostasis, 2008, 6(5): 820-829.
Zhang D, Ipse K, Raghavan N, et al. Metabolismus, pharmacokinetica et pharmacodynamica factoris Xa inhibitoris apixaban in leporibus[J]. Acta thrombosis et thrombolysis, 2010, 29(1): 70-80.
Ipse K, Luettgen JM, Zhang D, et al. Preclinical pharmacokinetica et pharmacodynamica apixaban, factor potens et selectivus Xa inhibitor [J]. Acta medicamentorum metabolismi et pharmacokineticorum Europaeorum, 2011, 36(3): 129-139.
Chemical structure





Rogatio18Qualitas Constantia Aestimatio inceptis quae probaverunt4et6incepta approbantes.

Provectus qualitas internationalis administrationis systematis solidum fundamentum in venditionibus posuit.

Qualitas vigilantia percurrit totum vitae cyclum producti, ut qualitatem et therapeuticum effectum curet.

Negotiis professionales regulares turmas sustinet qualitatem postulatorum in applicatione et adnotatione.


Korea Countec Bottled Packaging linea


Taiwan CVC Bottled Packaging Line


Italia CAM Board Packaging Line

German Fette Compacting Machine

Iaponia Viswill Traba Detector

DCS Control Room





