Rosuvastatin Calcium
| 瑞舒伐 他 汀钙 | Rosuvastatin Calcium | 147098-20-2 | In-Domus/CEP |
| RSM(Crystal) | 147118-40-9 | In-Domus | |
| RSM(Crude) | 147118-40-9 | In-Domus | |
| RSP | - | In-Domus | |
| RS-8 | 147118-36-3 | In-Domus | |
| RS-8-1 | 147118-37-4 | In-Domus | |
| TP-8 | 131466-61-0 | In-Domus | |
| TP-11 | 147118-35-2 | In-Domus | |
| TP-13 | 147118-39-6 | In-Domus | |
| RS-10 | 289042-12-2 | In-Domus | |
| RS-11 | 355806-00-7 | In-Domus |
Descriptio
Rosuvastatin Calcium (BANM, JAN, USAN) notum est Rosuvastatin in US.et concursus HMG-CoA inhibitoris reductase cum IC50 11 nM[1].Rosuvastatin Calcium potenter praecludit a-go-ire humanum gene (hERG) currentem cum IC50 195 nM, repolarizationis cardiacis retardatum, ac per hoc longas actiones potentiales durationes (APDs) et intervalla QT intervalla (QTc) emendavit[2].Rosuvastatin Calcium expressionem hERG maturitatis et commercii caloris incursus 70 (Hsp70) cum dapibus hERG minuit.Rosuvastatin Calcium efficacissimum est ad demissionem humilitatis densitatis lipoprotein (LDL) cholesterolum, triglyceridas, et C-reactivum dapibus campestribus[3].
Praeterea notitia de nominatione medicamentorum conventionibus: Internationalis Nomina nonproprietaria.
Praecipua Notitia: Drus.com database in BETA emissio internationalis est.Hoc significat adhuc sub evolutione et indiligentias continere potest.Non destinatur substitutus peritiae ac iudicii tui medici, pharmacopolae vel alius sanitatis professionalis.Non debet construi ad indicandum usum cuiuslibet medicamentorum in quacumque regione tutum, opportunum vel efficax tibi.Consule cum professionali sanitatis antequam ullum medicamentum accipias.
In US, Rosuvastatin (rosuvastatin systemica) membrum est status classium medicamentorum et adhibetur tractare Atherosclerosis, Maximum Cura, Maximum Cura - Familiale Heterozygoum, Maximum Cura - Familiale Homozygoum, Hyperlipoproteinemia, Hyperlipoproteinemia Type IIa - Elevatum LDL, Type Heterozygous, Maximum Cura - Familiale Homozygoum, Hyperlipoproteinemia, Hyperlipoproteinemia Type IIa - Elevatum LDL, Type Heterozygous IIb - Elevata LDL VLDL, Hyperlipoproteinemia Type III - Elevata beta-VLDL IDL, Hyperlipoproteinemia Type IV - Elevata VLDL, Hypertriglyceridemia et Praeventio Morborum cardiovascularium.
Background
Inhibitor selectivus, competitive HMG-CoA reductase, id est etiam antilipemica.
Repono
| Pulvis | -20°C | III annos |
| 4°C | Annis II | |
| In solvendo | -80°C | VI menses |
| -20°C | I mensis |
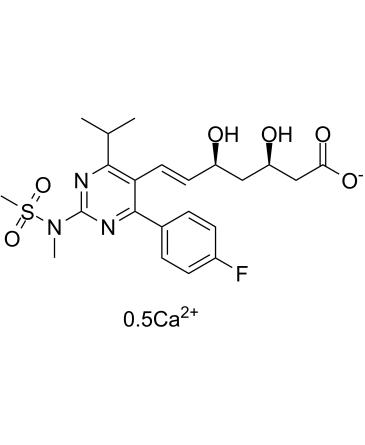
Chemical structure





Rogatio18Qualitas Constantia Aestimatio inceptis quae probaverunt4, et6incepta approbantes.

Provectus qualitas internationalis administrandi ratio solidum fundamentum venditio posuit.

Qualitas vigilantia percurrit totum vitae cyclum producti, ut qualitatem et therapeuticum effectum curet.

Negotiis professionalibus regularibus turmas sustinet qualitatem postulatorum in applicatione et adnotatione.


Korea Countec Bottled Packaging linea


Taiwan CVC Bottled Packaging Line


Italia CAM Board Packaging Line

German Fette Compacting Machine

Iaponia Viswill Traba Detector

DCS Control Room





