Velpatasvir(PVP)
Aliena Nomina
Velpatasvirum (Latin)
Velpatasvir (German)
Velpatasvir (French)
Velpatasvir (Hispanice)
Nomina generalia
Velpatasvir (OS: usan)
GS-5816 (IS).
UNII-KCU0C7RS7Z (IS)
Multi-ingrediens medicamentum velpatasvir:sofosbuvir/velpatasvir systemic
Notam nomina: Epclusa
Drug class(es): junctiones antivirales
Sofosbuvir/velpatasvir systemica adhibetur in curatione: Hepatitis C
sofosbuvir/velpatasvir/voxilaprevir systemic
Notam nomina: Vosevi
Drug class(es): junctiones antivirales
Sofosbuvir/velpatasvir/voxilaprevir systemica adhibetur in curatione: Hepatitis C
| 维帕他韦 | Velpatasvir | 1377049-84-7 | In-Domus |
| VLPM1 | 334769-80-1 | In-Domus | |
| VLPM2 | 1378388-16-9 | In-Domus | |
| VLP-5-Br | 1438383-89-1 | In-Domus | |
| VLP-5 | 1378390-29-4 | In-Domus | |
| VLP-10 | 1378391-45-7 | In-Domus |
Chemical Properties
| Repono | Copia apud -20°C |
| M.Wt | 883.0 |
| Cas No. | 1377049-84-7 |
| Formulae | C49H54N8O8 |
| Synonyma | GS-5816 |
| Solubilitas | ≥146.66mg/mL in DMSO (Opus ultrasonic et calefactio); Insolubilis in H2O' |
| SDF | Download SDF |
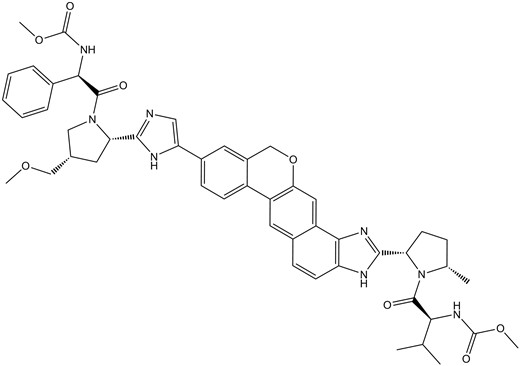
| CANONICI SMILES | O=C(N1C[C@@H](COC)C[C@H]1C2=NC=C(C3=CC=C(C(C=C(C=CC4=C5N=C([C@H) ]6N(C([C@H](C(C)' )C)NC(OC)=O)=O)[C@@H](C)CC6)N4)C5=C7)=C7OC8)C8=C3)N2)[C@H](NC(OC)= O)C9=CC=CC=C9 |
| Shipping Condition | Iudicium specimen solutionis: navis cum glacie caeruleo. Omnes aliae magnitudinis praesto: navis cum RT vel glaciali caerulea postulante. |
| General tips | Ad solutionem altiorem obtinendam, tubus 37°C calefacies et eam in balneis ultrasonicis aliquandiu excute. Solutio stirpe infra -20°C aliquot menses condi potest. |
Chemical structure





Rogatio18Qualitas Constantia Aestimatio inceptis quae probaverunt4et6incepta approbantes.

Provectus qualitas internationalis administrationis systematis solidum fundamentum in venditionibus posuit.

Qualitas vigilantia percurrit totum vitae cyclum producti, ut qualitatem et therapeuticum effectum curet.

Negotiis professionales regulares turmas sustinet qualitatem postulatorum in applicatione et adnotatione.


Korea Countec Bottled Packaging linea


Taiwan CVC Bottled Packaging Line


Italia CAM Board Packaging Line

German Fette Compacting Machine

Iaponia Viswill Traba Detector

DCS Control Room









