Ticagrelor
Background
Ticagrelor novus adversario receptoris P2Y12 est.
Ticagrelor relatum est ut effectus prothrombotici ADP in laminam contra receptorem P2Y12 inhiberent. Ticagrelor integram inhibitionem aggregationis ex vivo ostendit. Praeter Ticagrelor inhibitionem dosi dependens aggregationis in homine dependens. Praeter haec, Ticagrelor etiam viva voce, active, reversibly ostendit antagonistam obligare. Dissimiles inhibitores, Ticagrelor etiam retulit receptorem P2Y12 inhibere sine metabolicae transmutationis. Praeter haec, Ticagrelor est primum thienopyridinis anti-platelae, et maxime metabolum ab CYP3A4 et CYP2C19 [1][2].
Notae:
[1] Zhou D1, Andersson TB, Grimm SW. In vitro aestimatio potentiae medicamentorum medicamentorum cum ticagrelo interationes: cytochroma P450 reactionem phaenotypi, inhibitionis, inductionis et differentialis in motu. Medicamento Metab Dispos. MMXI Apr.
[2] Li Y1, Landqvist C, Grimm SW. Dispositio et metabolismi ticagrelori, novo receptore P2Y12 adversario, in muribus, muribus, et marmosetis. Medicamento Metab Dispos. 2011 Sep; 39(9):1555-67. doi: 10.1124/dmd.111.039669. Epub 2011 Iun 13 .
Descriptio
Ticagrelor (AZD6140) convertitur oralis receptor P2Y12 ad tractationem aggregationis platelet.
in Vitro
Ticagrelor maiorem inhibitionis adenosinae promovet 5 ..-diphosphate (ADP)-adductus Ca2+ emissio in platelis aliis nobis P2Y12R diluendis. Hic additus effectus ticagrelorum ultra P2Y12R repugnantiam partim est ut consequentia ticagrelorum inhibens vectorem aequilibrativae nucleoside 1 (ENT1) in platelis, ducens ad accumulationem adenosini extracellularem et activationem receptorum Gs-iunctarum adenosinorum A2A[1]. Cellulae B16-F10 commercium minutum exhibent cum platelis a ticagrelo-tractatis murium murium salinorum affectis comparatis[2].
In B16-F10 melanoma intravenosa et intrasplenica metastasis exempla, mures cum dosi ticagrelori orcis tractati (10 mg/kg) exhibet reductiones notatas in pulmone (84%) et hepatis (86%) metastases. Praeterea curatio ticagrelor meliorem salutem comparatis animalibus salinis affectis. Similis effectus observatur in exemplo 4T1 carcinomatis pectoris, cum reductionibus in pulmone (55%) et medulla ossis (87%) metastases quae sequuntur curatio ticagrelor[2]. Unius oralis administratio ticagrelorum (1-10 mg/kg) causat doses inhibitores effectus in aggregatione platelet. Ticagrelor, in summa dosi (10 mg/kg) signanter infert aggregationem in 1 h post dosing et inhibitionis apicem in 4 h post dosing observatur.
Repono
4°C, custodi a lumine, sub nitrogen conditum
*In solvendo: -80°c, menses VI; -20°C, 1 mensis (protegat a luce sub nitrogen conditum)
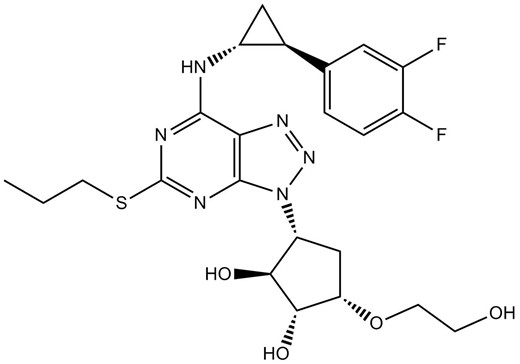
Chemical structure





Rogatio18Qualitas Constantia Aestimatio inceptis quae probaverunt4et6incepta approbantes.

Provectus qualitas internationalis administrationis systematis solidum fundamentum in venditionibus posuit.

Qualitas vigilantia percurrit totum vitae cyclum producti, ut qualitatem et therapeuticum effectum curet.

Negotiis professionales regulares turmas sustinet qualitatem postulatorum in applicatione et adnotatione.


Korea Countec Bottled Packaging linea


Taiwan CVC Bottled Packaging Line


Italia CAM Board Packaging Line

German Fette Compacting Machine

Iaponia Viswill Traba Detector

DCS Control Room










